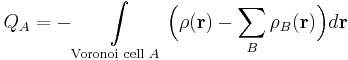Voronoi deformation density
Voronoi deformation density (VDD) is a method employed in computational chemistry to compute the atomic charge distribution of a molecule in order to provide information about its chemical properties. The method is based on the partitioning of space into non-overlapping atomic areas modelled as Voronoi cells and then computing the deformation density within those cells (i.e. the extent to which electron density differs from that of an unbonded atom).[1]
The VDD charge QA of atom A is computed as the (numerical) integral of the deformation density ∆ρ(r) = ρ(r) – ΣBρB(r) associated with the formation of the molecule from its atoms over the volume of the Voronoi cell of atom A:
The Voronoi cell of atom A is defined as the compartment of space bounded by the bond midplanes on and perpendicular to all bond axes between nucleus A and its neighboring nuclei (cf. the Wigner–Seitz cells in crystals). The Voronoi cell of atom A is therefore the region of space closer to nucleus A than to any other nucleus. Furthermore, ρ(r) is the electron density of the molecule and ΣBρB(r) the superposition of atomic densities ρB of a fictitious promolecule without chemical interactions that is associated with the situation in which all atoms are neutral.
Note that an atomic charge is not a physical observable. Nevertheless, it has been proven a useful means to compactly describe and analyze the electron density distribution in a molecule, which is important for understanding the behavior of the latter. In this connection, it is an asset of VDD atomic charges QA that they have a rather straightforward and transparent interpretation. Instead of measuring the amount of charge associated with a particular atom A, QA directly monitors how much charge flows, due to chemical interactions, out of (QA > 0) or into (QA < 0) the Voronoi cell of atom A, that is, the region of space that is closer to nucleus A than to any other nucleus.